SAN FRANCISCO, Aug. 11, 2020 /PRNewswire/ -- Darmiyan, Inc., is pleased to announce the successful completion of its third-party validation study aimed at blind-testing and validating BrainSee (Darmiyan's proprietary, patent-protected technology), in predicting the cognitive future (prognosis) of mild cognitive impairment patients in the actual clinical setting. The third-party investigators were from Stanford University, Baycrest Institute, Huntington Medical Research Institutes (HMRI), University Health Network (UHN), Knight Alzheimer's Disease Research Center (ADRC) at Washington University in St. Louis, and GERAS Hamilton Health Sciences (HHS). The Canadian trial sites were coordinated by CABHI (Centre for Aging & Brain Health Innovation) following a CABHI-I2P2 (Industry Innovation Partnership Program) grant that was awarded to Darmiyan in 2018.
"Darmiyan's AI, machine learning algorithm using unique MRI microscopic voxel analysis with macroscopic input has generated a very sensitive and specific five year prognosis for patients presenting with amnestic MCI," noted Jamshid Ghajar, MD, PhD, FACS, Moghadam Family Director of Stanford Medicine Brain Performance Center. "This technology has high test-retest reliability and can be applied to any clinical grade MRI which is a very useful clinical tool to assist doctors advising patients with early memory complaints."
Validating BrainSee on Both Clinical-grade and Research-grade Data
The current study showed that BrainSee performs with as high accuracy on clinical-grade data (from patients who present to clinics and hospitals) as it had previously performed on research-grade data (from patients who volunteer for research or clinical trials). This makes BrainSee the first cutting-edge solution that can be easily integrated in any community clinical setting around the world, not just the high-end academic institutions.
A blind retrospective analysis was previously conducted on 411 amnestic mild cognitive impairment (aMCI) patients with research-grade input data (MRI: 3D T1, T2, DTI; plus MMSE & CDR) reporting 90+% performance accuracy. In the current third-party validation study, external investigators blind-tested BrainSee on 107 new patients with clinical-grade input data (routine clinical T1, T2, DWI; plus MMSE & estimated CDR from clinician notes).
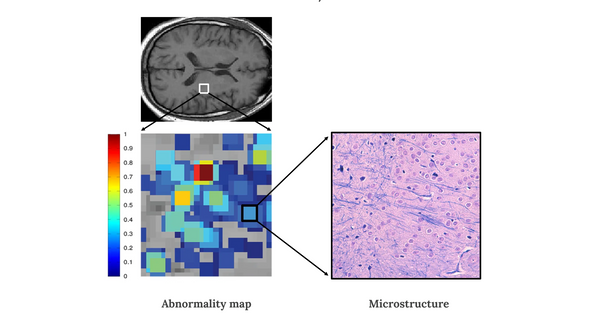
While all 107 patients held the same clinical diagnosis label of aMCI, BrainSee visualized and quantified microstructural differences that existed in each patient's brain, and predicted if they would convert to Alzheimer's dementia within 5 years (converter) or stay stable/ improve (non-converter). Investigators compared BrainSee's predictions against the actual clinical diagnosis five years later. The overall predictive accuracy of BrainSee was reported as 91% (Sensitivity 90%, Specificity 92%). "Such an accurate and non-invasive prognostic tool is non-existent in the market today," noted the US investigators.
The current study also included a separate analysis for evaluating BrainSee's test-retest reliability on 84 additional aMCI patients who were recruited to get two brain MRI scans on the same day: one clinical-grade and one research-grade. The test-retest reliability (consistency) of BrainSee was reported very high, with a correlation coefficient of 99.5%.
With this external validation data, the total number of aMCI patients tested by BrainSee has reached 602.
Novel Technological Advantages
"While reading brain PET scans at Stanford & NYU hospitals as a radiologist, I imagined the day when we could help clinicians visualize and evaluate brain health more comprehensively and objectively while posing less discomfort to patients. That day has come now and Darmiyan's BrainSee technology can finally bring clarity to the field of Alzheimer's through visualizing brain tissue microstructure for doctors. Darmiyan's Virtual Microscope technology unlocks the enormous informative potential of the currently underutilized brain MRI scans," said Darmiyan's Chief Medical and Technology Officer, Kaveh Vejdani, MD. "The huge advantage of MRI modality over PET is that it is safer (no radiation exposure), faster, more cost-efficient, non-invasive (does not require radiotracer injection), and much more widely available throughout the world."
According to Dr. Michael G. Harrington, FRCP, Scientific Director of Neuroscience, HMRI, "Objective measures such as Darmiyan's BrainSee that can predict cognitive decline are strongly needed to recognize and monitor potential therapies."
Meeting Today's Clinical Needs
David J. Mikulis, MD, Professor and Director of the JDMI (Joint Department of Medical Imaging) Functional Neuroimaging Research Lab, UHN, noted: "I was impressed by the potential of this breakthrough technology. All study investigators are optimistic that Darmiyan's solution will be successful providing a much needed predictor of disease progression. It may therefore fill a significant diagnostic gap highly valued by patients, clinicians, and clinical researchers."
"With the great help of our clinical trials sites in the US and Canada, we demonstrated that BrainSee can well integrate in the clinical workflow without imposing any limitations, such as specific MRI protocol, in the process of monitoring MCI patients," said Darmiyan's CEO Padideh Kamali-Zare, PhD. "These data support our vision to make BrainSee readily accessible for all people worldwide — addressing the dire need for accurate diagnosis of Alzheimer's disease and prognosis of mild cognitive impairment in clinics and clinical trials. Localizing and quantifying early signs of brain cell distortion, in a way that is agnostic to specific protein-based biomarkers such as amyloid or tau, is key to understanding the disease pathology early on in its process and providing personalized treatments suitable for each Alzheimer's patient in the future."
About Darmiyan
Darmiyan was incorporated in September 2016 and became a YC-backed company in Summer 2017. Prior to the current funding round, Darmiyan had raised $4.5M in equity financing and government grants. The company has won numerous awards and recognitions including the TEDMED Hive Innovator in 2018 and CABHI's I2P2 & innovation awards in 2018 & 2019, respectively.