San Francisco, CA, May 24, 2021: Today the American Society of Neuroradiology published Introducing BrainSee, a summary of study results presenting the 91 percent accuracy rate of the new AI-powered and patented technology by Darmiyan Inc. that detects Alzheimer’s disease at early stages of disease development. According to the publication made public at ASNR's annual meeting, subtitled, A Novel, MRI-based Virtual Microscope Technology for Non-invasive Prognosis of Amnestic MCI in Clinics and Clinical trials, leading experts in the US and Canada confirmed the high test, retest reliability of the technology available for research on early detection of Alzheimer’s related mild cognitive impairment through a non-invasive method. Investigators noted that BrainSee is due to facilitate development of therapeutics for mild cognitive impairment and AD that can fill the clinical gaps.
“I look forward to making widely available BrainSee, an imaginative new method for noninvasively illuminating and detecting Alzheimer’s at stages where intervention might be possible,” said Dr. Alexander Mark, Medical Director MedStar Imaging Network Rockville and Chevy Chase. BrainSee by Darmiyan, a California-based company, combines the latest in computational neuroscience and digital health technologies with medical imaging to provide a unique enterprise software solution to address mild cognitive impairment before dementia.
"Alzheimer’s disease is the leading cause of dementia, with 50 million people suffering from it worldwide. Three times as many, roughly 150 million people, worry that their sudden onset of memory loss or cognitive impairment is an early symptom of Alzheimer’s disease. In almost a third of worried patients, it’s not Alzheimer’s. We focus on extracellular space because it’s a frontier rich with information about the unseen early stages of mild cognitive impairment. Our goal with BrainSee is to help doctors provide reassurance and answer questions about disease progression: Will patients stabilize or improve? What time window do we have for planning? ” Dr. Padideh Kamali-Zare, CEO and Founder of Darmiyan, said.
“Darmiyan’s technology outperforms current diagnostic options and offers new hope for physicians who treat families impacted by Alzheimer’s,” commented Kaveh Vejdani, MD, Co-founder and Chief Medical and Technology Officer, who as a specialist in nuclear medicine envisioned and developed BrainSee because he saw how painful it was for patients to endure invasive health diagnostics.
“BrainSee unlocks value from brain MRIs, by providing objective measures for doctors’ prognosis of Alzheimer’s. With FDA approval, Darmiyan will facilitate better patient monitoring and precision medicine,” Lawrence Tanenbaum MD, CTO, Director of Advanced Imaging RadNet added.
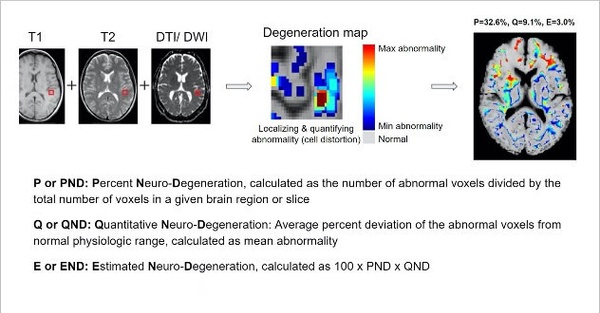
BrainSee is high accuracy and consistency has now been recognized in influential journals on both neuroradiology and disease prevention as an aMCI to AD prognosis tool.
Nate Bergman, DO, Chief Scientific Wellness Officer at Ohio-based Kemper Cognitive Wellness Center, and others have begun to secure Darmiyan analysis of MRIs. During his podcast interviewing Vejdani among leading thinkers on Alzheimer’s, Dr. Bergman noted how Darmiyan can help doctors screen for a “big problem” with models of cell distortion. He later observed, "predictive neuroimaging analytics will greatly help us focus resources and interventions on those that are most at risk of developing Alzheimer's disease."
“I am impressed with the Darmiyan team and their technology performance,” observed Dr. Alexandria Papaioannou, MD, Geriatrician, Hamilton Health Sciences, “their solution, which uses clinical grade MRI, and cognitive testing can improve clinicians’ workflow and how patients are monitored.”
By making prognosis of Alzheimer’s in the aging population possible, Darmiyan will help organizations plan better to avoid the costly risks of the future -- what Barron’s reporters called “the other pandemic.” Identifying mild cognitive impairment and monitoring dementia is destined to guide better management of comorbidities, lower hospitalization rates, and higher quality of life for Medicare patients.
Based in San Francisco, California Darmiyan was incorporated in September 2016 and backed by Y-Combinator (YC) in Summer 2017. The Company has won numerous awards and recognitions including the TEDMED Hive Innovator in 2018 and CABHI Innovation Award in 2019 and Fortune 40 Under 40 for healthcare in 2020. Darmiyan's most recent funding in 2020 was led by the global pharma giant Eisai with participation of YC and IT-Farm. Darmiyan’s proprietary technology is patented in the US; patents are pending in Europe, Japan and China.